Diving is an excellent activity with the potential for one-of-a-kind experiences you can only get in an aquatic environment. Our land-loving bodies, however, can react negatively to diving if we are not careful. We are drilling down the essential know-how of diving so you can enjoy it to the fullest.
Pressure changes; your body needs to adapt.
Diving is made easy to understand and more accessible: dive algorithms
Pressure changes; your body needs to adapt.
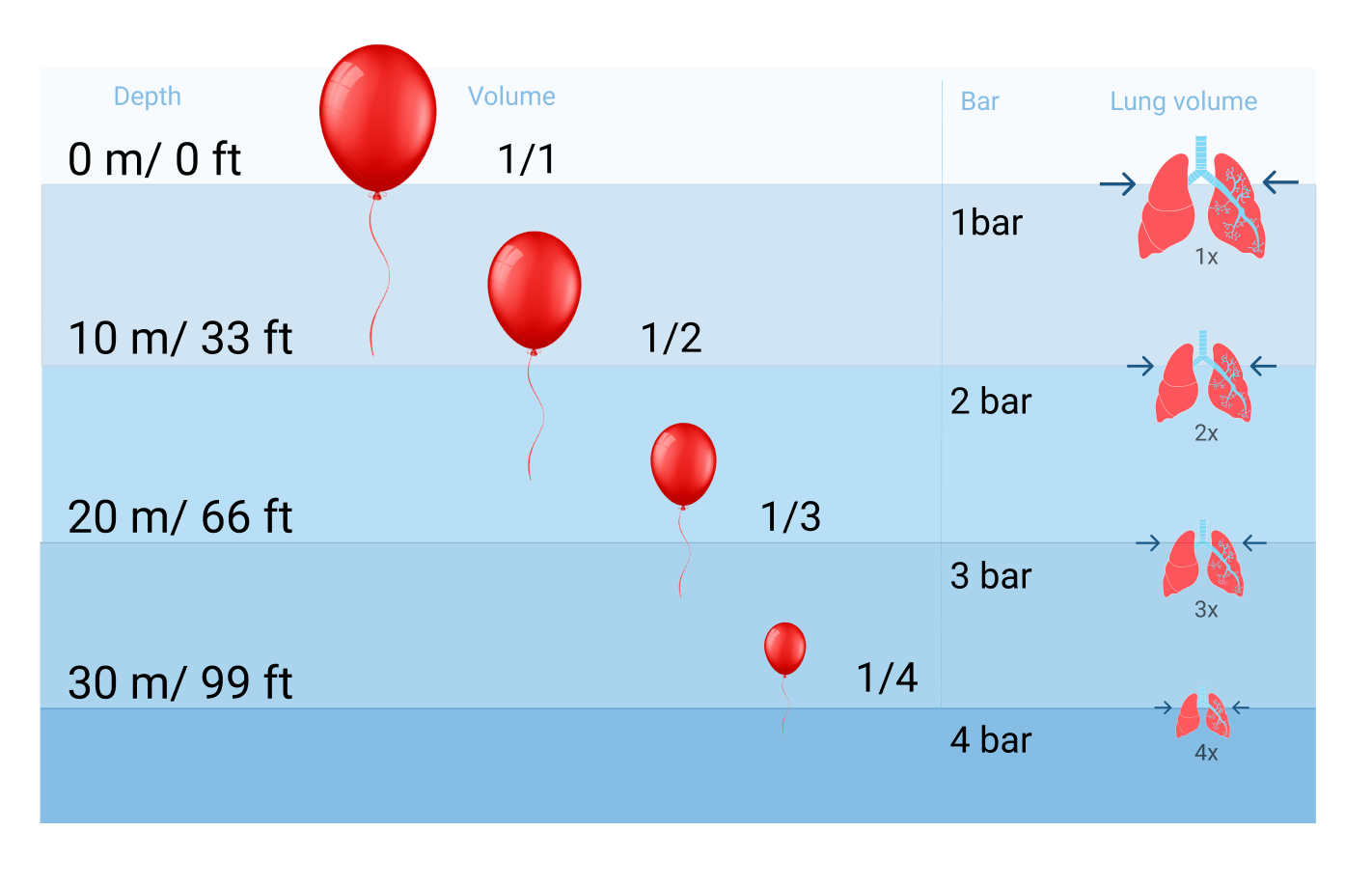
There are constant fluctuations in the surrounding pressure while we hike or dive into the ocean. Pressure changes 1bar/14.7 PSI for every 10 meters and it increases much faster underwater because water is denser than air. The pressure we face underwater, also called ambient pressure, is caused by the water's weight. The deeper we dive, the greater the ambient pressure will become. Ten meters down is already twice the pressure at the surface.
Discomfort in the ears when taking off in an airplane is also felt just diving down to the bottom of a pool that is three meters deep. When a diver descends, the surrounding water pressure grows. The pressure change underwater will affect spaces that contain air, such as your ears, sinuses, BCD, and mask. But the most significant impact is on your circulatory and respiratory systems and the latter need to be taken seriously because they can lead to major health risks.
What happens after you immerse into water or hike up a mountain?
Our bodies are full of dissolved gases from the air we breathe. Our bodies actively use oxygen for us to function. Other gases, the so-called inert gases like nitrogen, are not used by our bodies but are stored in blood and tissues. While diving, the pressure increases, and our body is exposed to a higher absorption rate of nitrogen stored in our tissues. The amount of inert gas dissolved in our bodies depends on the ambient pressure. Why is it that e don't feel this effect? Because our body is mainly made up of liquids, we are not exposed to pressure. Nevertheless, we feel it in our ears and sinuses because of the air trapped in them.
When we ascend from a dive, the ambient pressure is reduced and the dissolved nitrogen needs to come out (off-gas). We have no problems as long as nitrogen comes out slowly and in a controlled manner without significant pressure differences. If the
pressure is released too fast; the nitrogen will come out too fast and cause DCS, also known as decompression sickness, or "the bends."
The amount of gases dissolved in our bodies depends on the ambient pressure around us. That means that every gas has a specific partial pressure, and the combined pressures of the gases in our bodies stay in equilibrium with our environment. Your body is fully saturated with gases at the elevation where you can be found for an extended time. Here are two scenarios that will explain the changes in your body:
- If you hike up a mountain, air pressure drops, making your body hold less gas. Your tissues are at this point supersaturated relative to the new ambient pressure. Our bodies release gas through diffusion and breathing to get back to equilibrium, also known as off-gassing.
- When you go down to sea level and then underwater, you increase your bodies' pressure, allowing more gas to be carried by blood and tissues. Again, to equalize the pressures, your body takes on more dissolved gas from the air you breathe. This is called on-gassing.
Does the same thing happen when you ascend from a dive?
If we come up from a dive too quickly (therefore, dropping ambient pressure), the natural off-gassing mechanisms are overloaded. The dissolved gas in our bodies comes out of the solution too fast, forming bubbles that can cause decompression sickness or DCS. There are different stages and forms of DCS, and symptoms can range from minor joint pain and skin irritation to severe nerve damage and death. For a diver with DCS, the symptoms may already start while still underwater, or it may take several hours after surfacing. In some cases, the symptoms may not show for several days. Nevertheless, most cases are treatable with, for example, recompression chamber treatment (hyperbaric oxygen treatment).
Diving is made easy to understand and more accessible.
Over the decades, dive algorithms were incorporated into dive computers to calculate how long we can stay underwater with a limited risk of getting DCS. A dive computer knows your dive history and calculates the safety limits in real-time by considering the following metrics: depth, time, gas mix, and personal factors (if applicable).
What is a dive algorithm?
A dive algorithm is a theoretical mathematical formula and does not measure your body's actual physical state while diving. Everybody is different and no dive computer (to this day) can measure the amount of inert gas in every body tissue. Every dive computer has a level of conservatism built in to minimize the risk of DCS and by changing your personal settings, you can add or remove safety margins to your dive algorithm.
What does a dive algorithm do?
Algorithms are designed to give you a safe estimate of how long you can stay at different depths without developing the risk of DCS by considering time/depth/dissolved gas. Some algorithms provide longer dive times at the cost of a higher likelihood of DCS, while others limit the dive time to add a safety margin to your dive.
They are used in dive computers based on how inert gases are absorbed and dissolved into and from the diver’s tissues. There are two decompression models most commonly used: the gas model, or the Haldane model, and then the bubble model, known as VPM and RGBM.
- The first one is based on J.S Haldane's work and according to his theory, the body is grouped into theoretical tissue compartments, absorbing and releasing inert gas at different rates. This theory is based on avoiding bubble formation by controlling the absorption and release in different theoretical tissue compartments. One commonly used algorithm following the principles of the gas model is the Bühlmann ZHL- 16C.
- The second commonly used decompression model is based on the assumption that bubble formation will always be present and the key is to control the size of the formed bubbles. Suunto Fused™ RGBM 2 has been developed with Dr. Bruce Wienke to combine the benefits of the VPM model with Dr. Wienke’s latest full RGBM work.
What should you do?
The important takeaway of this introduction is that every diver and dive are different, so are the underlying assumptions for this incredible sport. In the end, you as a diver will decide what your safety margins are and what theoretical model you want to use for your dives, and those choices will be based on your training, your experience, and eventually, your preferences. Take your time to discover the underworld safely and you will not be disappointed.